Which Of The Following Best Explains What Is Happening When An Atom Emits Light?
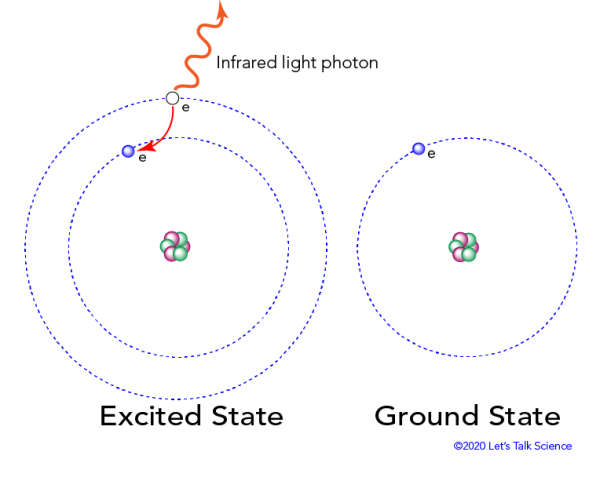
Which of the following best explains what is happening when an atom emits light?. Circle the letter that best explains why cells containing chlorophyll are green. When an atom emits light it decays to a lower energy state. Describe what happens when the frequency of the light shining on an object resonates with the objects natural frequency.
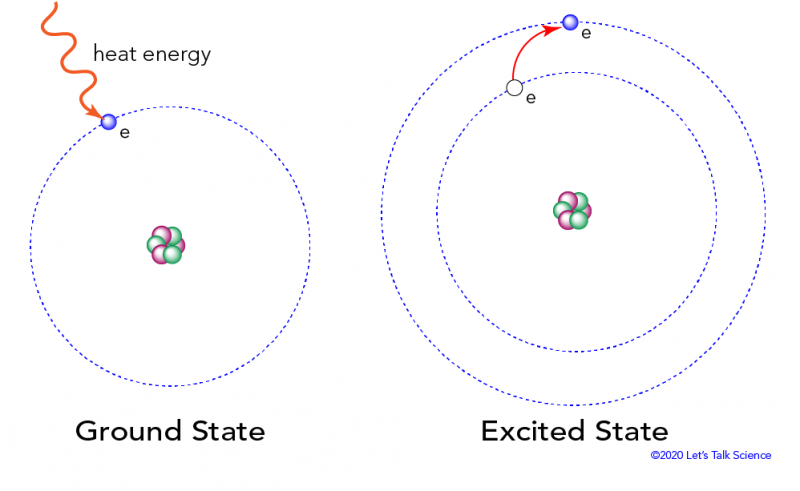
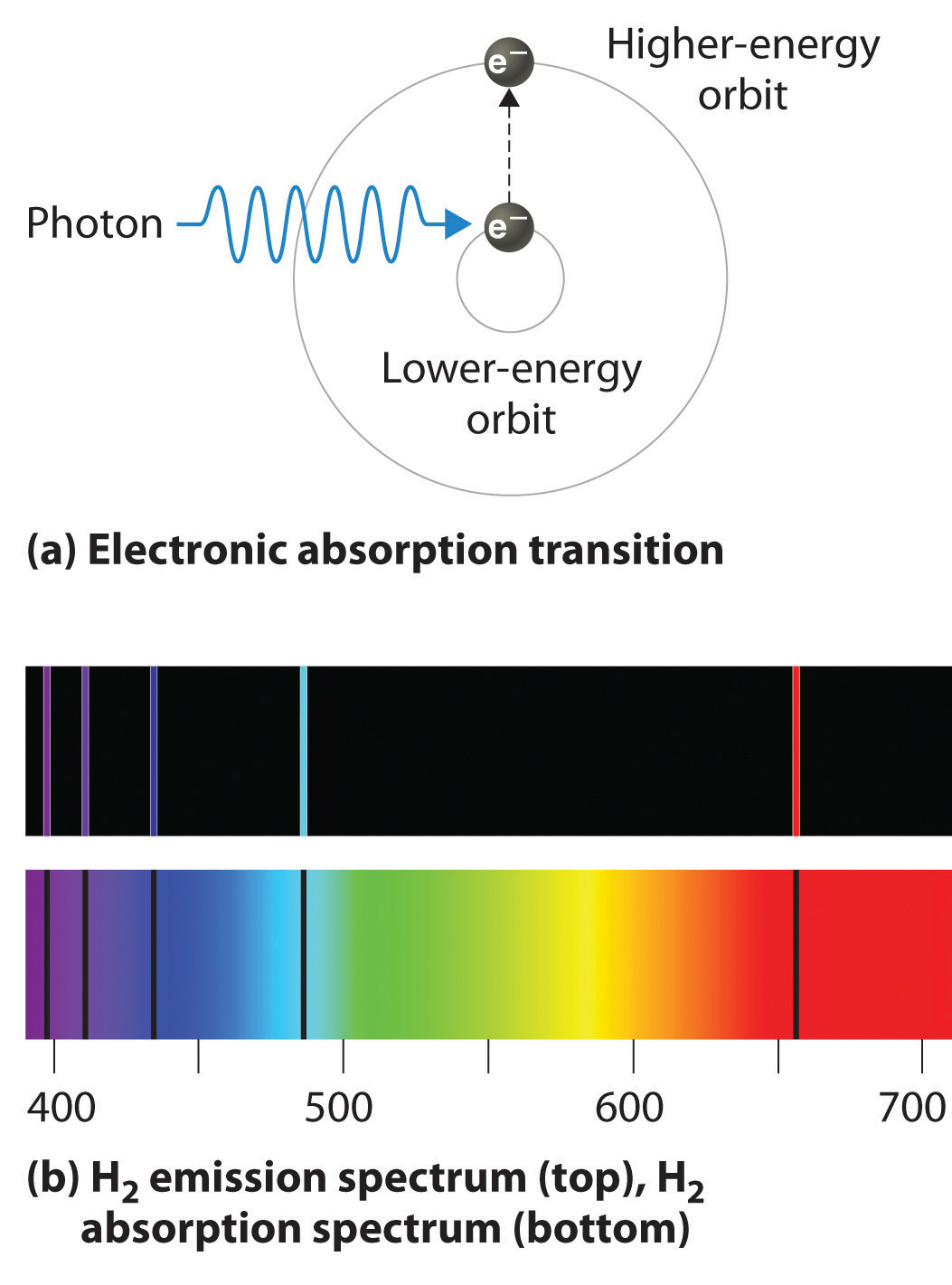
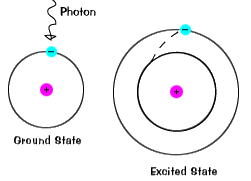
1 The atom stores the energy for later use. Zinc sulfide ZnS doped with silver and aluminum emits blue light. When an atom absorbs light it is excited to a higher energy state.
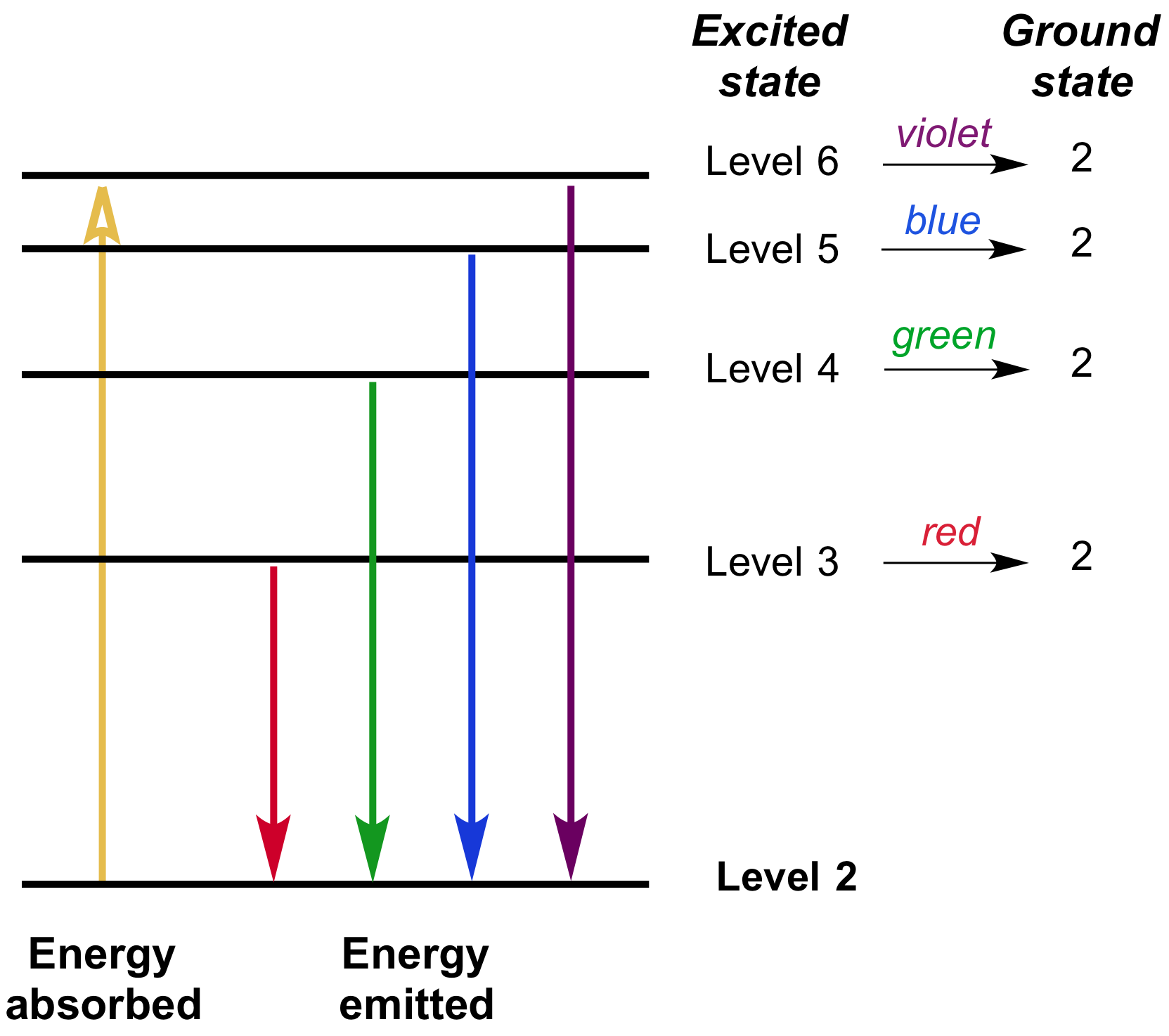
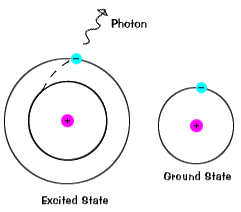
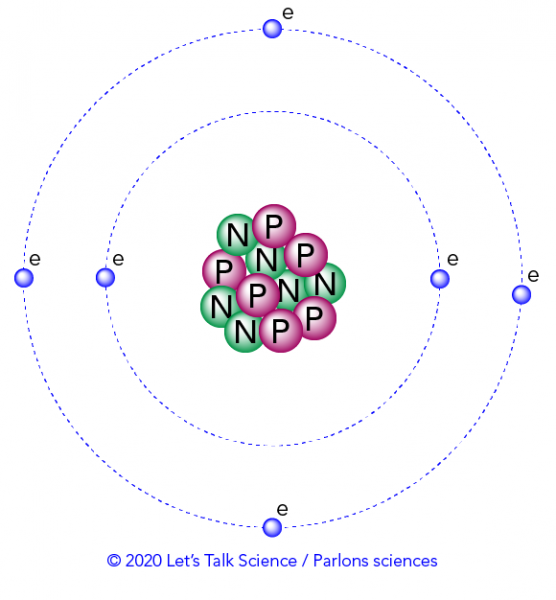
Which of the following best explains what is happening when an aton emits light. An atom of some element emits orange light for a transition from n4 to n3. An atom emits light when one of its electrons falls from a higher energy level to a lower one.
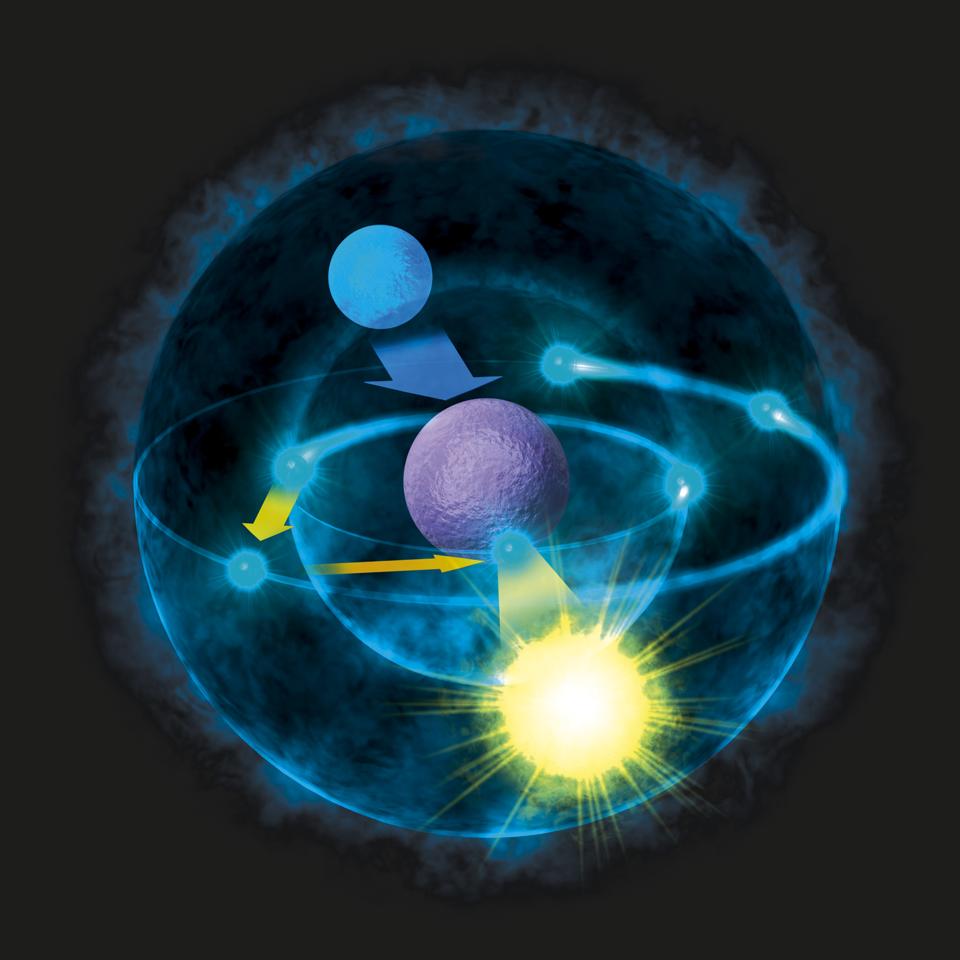
An electron is dropping from a higher to a lower energy level with the difference in energy between the two being emitted as light energy. When something excites an atom such as a collision with another atom or a chemical electron an electron may absorb the energy boosting it up to a higher-level shell. Heat energy is speeding up the orbit of the electrons and the resulting Doppler shift causes light to be emitted from the electrons.
Which of the following best explains what is happening when an atom emits light. 3 The extra energy increases the speed of the electrons in their orbitals. Which of the following best explains what is happening when an atom emits light.
An atom emits anand. Green light is emitted on going from n5 to n4. A phosphor is a mineral that absorbs UV light and converts it to visible light.
The boost is short-lived however and the electron immediately falls back down to the lower level emitting its extra. Neon gas reacts with oxygen in the air which causes the emission of light.
When something excites an atom such as a collision with another atom or a chemical electron an electron may absorb the energy boosting it up to a higher-level shell.
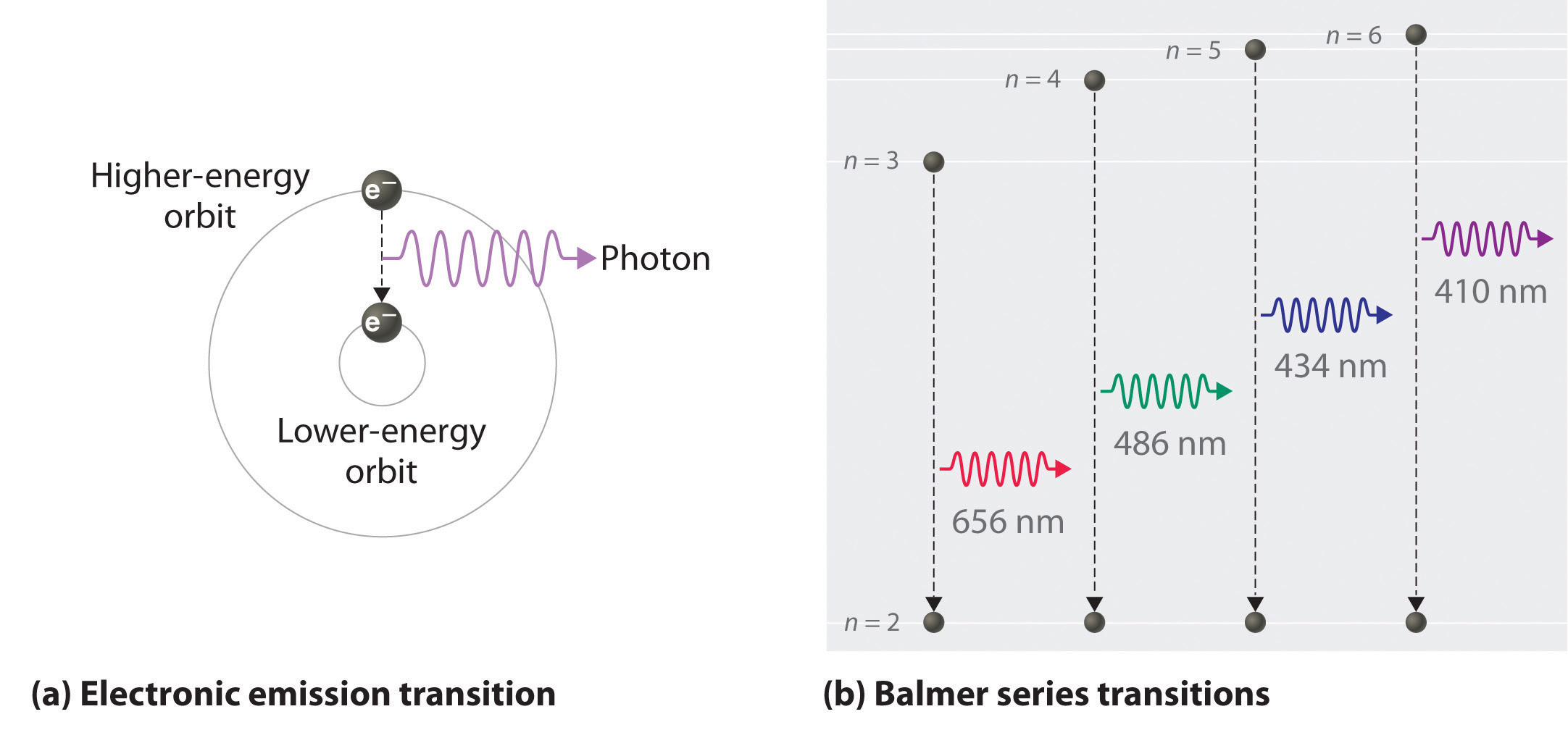
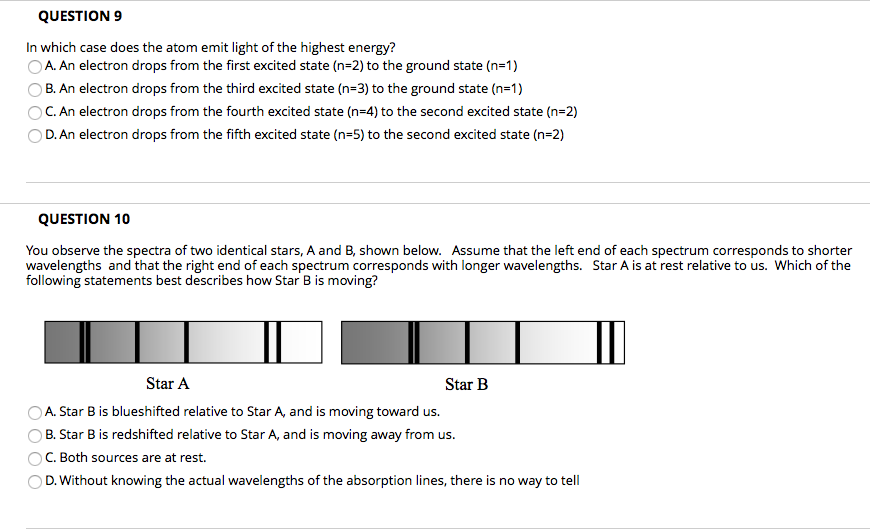
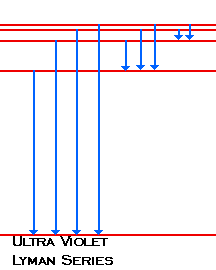
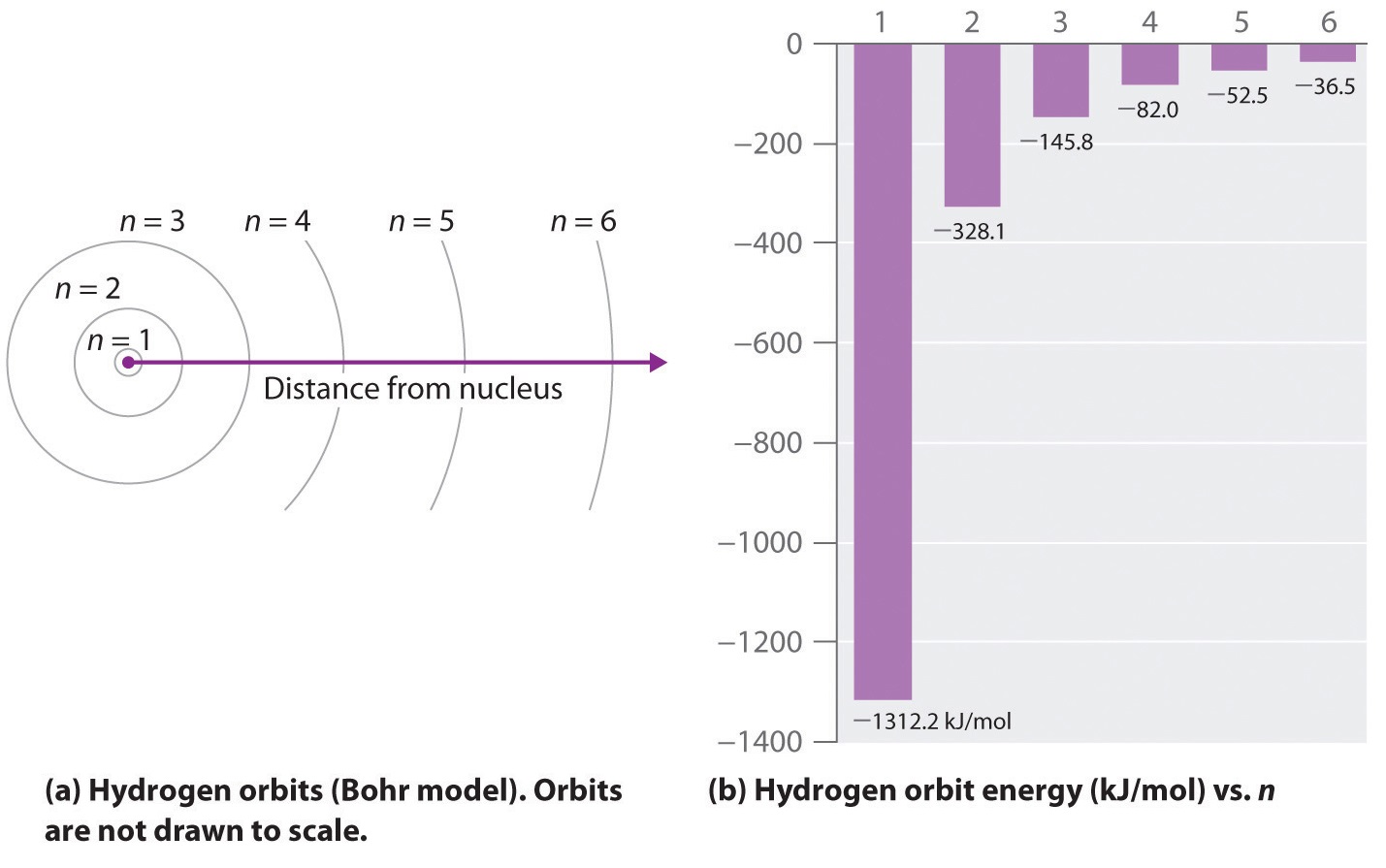
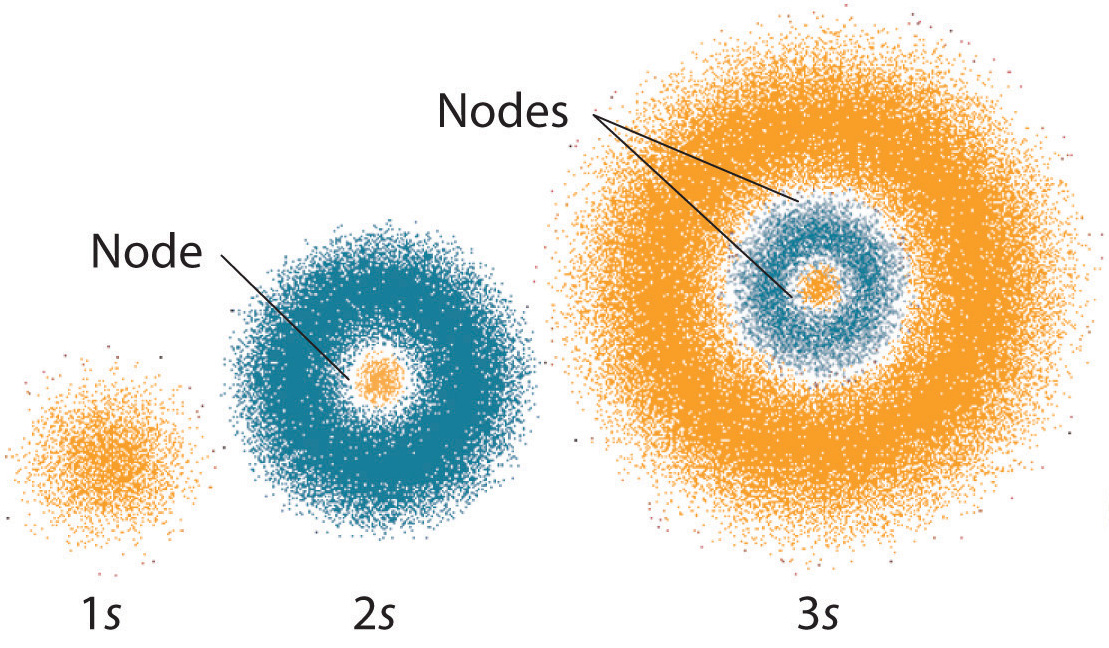
Heat energy is speeding up the orbit of the electrons and the resulting Doppler shift causes light to be emitted from the electrons. Which of the following best explains what is happening when an aton emits light. The photon or particle of light contains the energy that the electron loses. They absorb green light. The boost is short-lived however and the electron immediately falls back down to the lower level emitting its extra. Which of the following best explains what is happening when an atom emits light. The Energy States of the Hydrogen Atom If white light is passed through a sample of hydrogen hydrogen atoms absorb energy as an electron is excited to higher energy levels orbits with n. An atom emits light when one of its electrons falls from a higher energy level to a lower one. Heat energy is converting a neutron into a proton and an electron which is ejected into the orbit of the nucleus releasing light energy e.
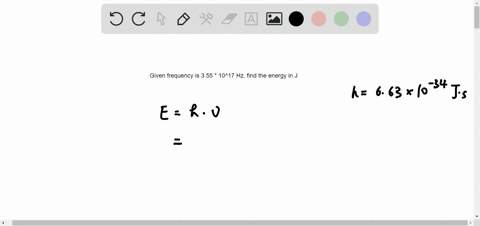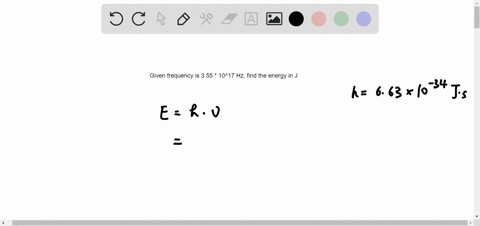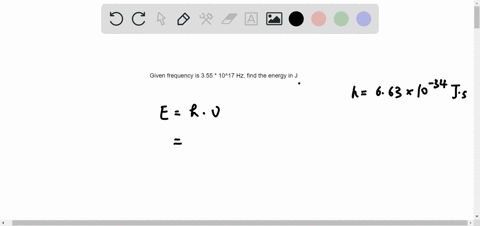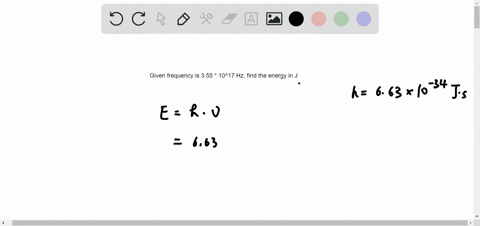
B A proton is undergoing a nuclear change in the nucleus and is emitting a high energy light wave in the process. An electron is dropping from a higher to a lower energy level with the difference in energy between the two being emitted as light energy. Which of the following best explains what is happening when an atom emits light. What is the energy of a photon of blue light that has a wavelength of 453 nm. Ultraviolet light excites the neon gas to produce emitted white light. Which of the following best explains what is happening when an atom emits light. A proton is undergoing a nuclear change in the nucleus and is emitting a high energy light wave in the process.








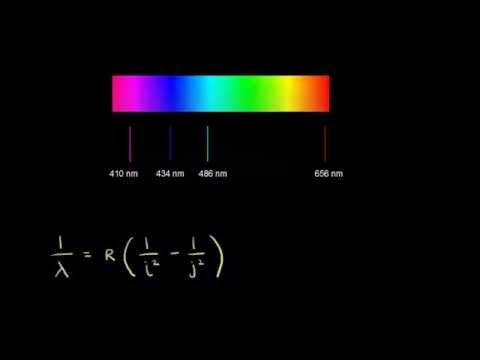













Post a Comment for "Which Of The Following Best Explains What Is Happening When An Atom Emits Light?"